Drug-Diagnostic Co-Development: Integrated Solutions from Discovery to Launch
Precision medicine is a new medical model that "tailors" treatment plans for patients based on their individual genomic and molecular characteristics. It is particularly suitable for complex diseases such as cancer—because the same disease may be driven by different genes, and different diseases may share the same mutations. Precision diagnosis guides personalized treatment by identifying these molecular-level similarities and differences. In this system, precision diagnosis is the prerequisite for precision treatment, while companion diagnostics is a key supporting technology. It screens patients suitable for targeted therapy by detecting specific biomarkers, thus truly achieving a precise closed loop from diagnosis to treatment.
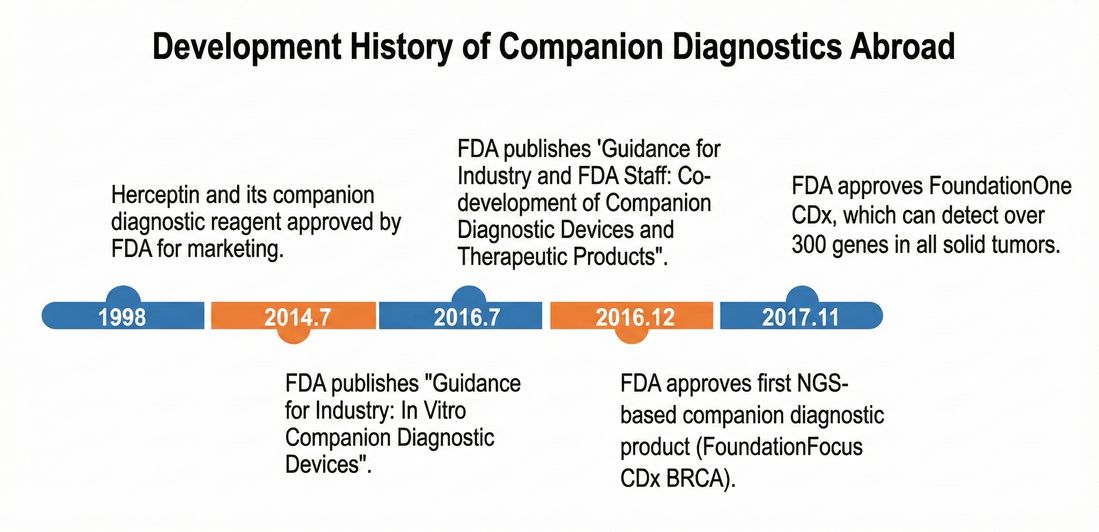 Figure 1 The Development History of Companion Diagnostics
Figure 1 The Development History of Companion Diagnostics
(Creative Enzymes Diagnostic)
With the support of Creative Enzymes Diagnostic, you can efficiently coordinate the development processes of drugs (Rx) and diagnostics (Dx). Our team of medical and scientific experts covering all therapeutic areas will provide comprehensive support to ensure that your companion diagnostic kits are approved in sync with treatments and accelerate their time to market. Whether you plan to adopt the laboratory-developed testing (LDT) or in vitro diagnostic (IVD) pathway, our deep technical expertise, flexible strategic planning capabilities, and profound scientific insights can help you determine the optimal path and achieve value together.
We ensure that companion diagnostic kits and drug development progress simultaneously and collaboratively through a well-structured and phased cooperation process. (The following is a diagram of key milestones; the actual process will be customized according to the project.)
| Stage | Key areas of collaboration | Key Activities and Deliverables |
|---|---|---|
| Early Discovery | Develop preclinical detection methods based on drug targets. | Deliver the research protocol to support pharmacodynamics and biomarker exploration. |
| Clinical Translation | Transform the RUO method into a stable assay suitable for clinical trials. | Complete analytical method lock-in. |
| Pivotal Clinical Trials | Develop a prototype companion diagnostic kit for pivotal clinical trials. | Execute clinical sample bridging studies to ensure consistency between clinical data and future commercial kit results. |
| Industrialization and Validation | Complete process transfer, large-scale production, and comprehensive validation of the kit. | Provide a complete analytical performance validation report to support registration. |
| Registration and Submission | Prepare and submit registration documentation for the kit, and simultaneously build the supply chain. | Lead regulatory submissions and obtain kit market approval. |
| Commercialization | Ensure continuous and stable product production and supply. | Achieve commercialization and post-market support for the product. |
We understand that different pharmaceutical companies have different strategic needs and resource allocations, therefore we offer highly flexible cooperation models:
Creative Enzymes Diagnostic offers an integrated companion diagnostics solution from early-stage R&D to commercialization, seamlessly collaborating with your drug development to accelerate the delivery of precision medicine products.
Contact our business development team today to discuss your specific project needs!